Ethanoic Acid and Sodium Hydroxide
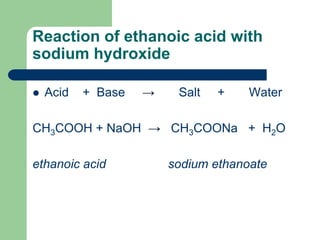
8600 Rockville Pike Bethesda MD 20894 USA. Acetic acid CH3COOH will react with sodium hydroxide NaOH to produce sodium acetate CH3COONa and water.

Net Ionic Equation For Naoh Ch3cooh Strong Base And Weak Acid
The results of the experiment are shown.

. The reaction of Ethanoic acid with a base Ethanoic acid undergoes a neutralisation reaction with a strong base like sodium hydroxide to form salt and water. Reaction of ethanoic acid CH 3 COOH with sodium Na. But ethnaote ion is not stable in the water and hydrolysis the water and release hydroxyl ions.
The chemical equation of the reaction is as follows. Sodium ethanoate which is produced when ethanoic acid reacts with sodium hydroxide finds uses in the textile and food industries as a neutralization agent preservative and a mild. This video with the help of flash animations shows and explains how you can determine the concentration of vinegar ethanoic acid using a standard solutio.
Sodium hydroxide solution and ethanoic acid Ammonia solution and hydrochloric acid Ammonia solution and ethanoic acid Results. The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid. The IUPAC name of.
What happens when a piece of sodium metal is added to ethanol and ethanoic acid reacts with sodium hydroxide chemical equations of the reactions. As we know ethanoic acid is a weak organic acid and when it reacts with sodium metal it results in the formation of sodium ethanoate or commonly called sodium acetate and the liberation of. The reaction of ethanoic acid with sodium metal results in the formation of sodium ethanoate or commonly known as sodium acetate and hydrogen gas is liberated.
Despite being weak ethanoic acid interacts with any base to generate one molecule of salt and one molecule of water. The unbalanced chemical equation that describes this neutralization. Running acid into the alkali For the first part of the graph you have an excess of sodium.
For reactions involving ethanoic acid or ammonia the measured enthalpy change of neutralisation is a few kilojoules less exothermic than with strong acids and bases. CH 3COOHNaOH sodium ethanoateCH. The balanced chemical reaction between ethanoic acid and sodium metal is shown below.
Acetic acid ethanoic acid and Sodium hydroxide reaction CH3COOH NaOH 1369 views Mar 17 2020 8 Dislike Share Heshan Nipuna 306 subscribers Acetic acid reacts. Add sodium hydroxide solution 04 M to ethanoic acid and hydrochloric acid. Ethanoic acid is a weak organic acid that combines with sodium metal to generate sodium ethanoate CH 3 COO-Na also known.
National Library of Medicine. When same amount mol of sodium hydroxide and ethanoic acid are mixed mixture contains sodium ethanoate. Strong bases are considered.
Sodium ethanoate dissociate completely in the water to ethnaote ion and sodium ion. The hydroxide ion makes sodium hydroxide a strong base which reacts. Do this by following the procedure in steps 5 6 7 and 8 but using sodium hydroxide instead of sodium.
National Institutes of Health. Chemical properties Sodium hydroxide is completely ionic containing sodium ions and hydroxide ions. N a s H 2 O l N a H.
Ethanoic acid is commonly known as acetic acid. Solution a When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed. Well take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base.
Ethanoic acid for instance interacts with sodium hydroxide.

How To Balance Naoh Ch3cooh Ch3coona H2o Youtube

List Two Chemical Properties On Basis Of Which Ethanol Ethanoic Acid

Naoh Ch3cooh Or Hc2h3o2 Nac2h3o2 H2o Balanced Equation Acetic Acid Or Ethanoic Acid Naoh Balance Youtube

Comments
Post a Comment